Intrathecal Drug Delivery to the CNS
Mechanistic Study of the Bio dispersion in Human Spine Heading link
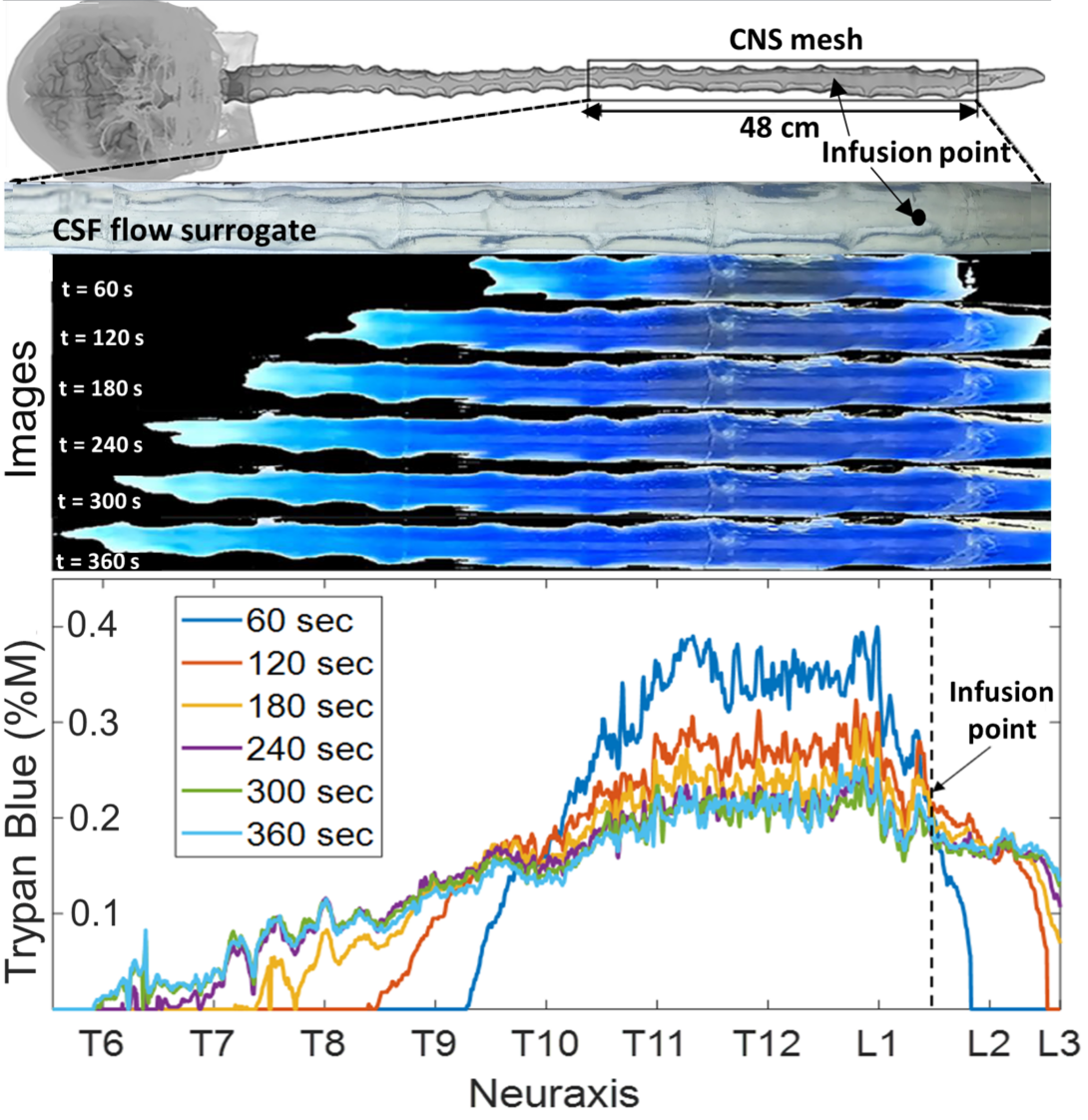
Mechanistic Study of the Biodispersion in Human Spine
Ayankola Ayansiji and Andreas Linninger
To understand how drugs are dispersed in the human central nervous system, we conducted an extensive in vitro study of bio-dispersion experiments in a subject specific anatomically accurate model of the human spinal subarachnoid space. Intrathecal drug delivery was studied using subject-specific deformable phantom models of the human central nervous system that we manufactured so that tracer infusion could be realistically replicated in vitro over the entire physiological range of pulsating cerebrospinal fluid (CSF) amplitudes and frequencies. Our experiments show that high volume infusions can overcome the perceived limitation of IT administration. A Distributed Mechanistic Pharmacokinetic (DMPK) model, which quantifies the tracer’s bio dispersion alongside uptake (lipophilicity, enzymatic decay, etc.) was then developed and used to back up the experimental data.
Intrathecal Drug Delivery to the CNS Heading link
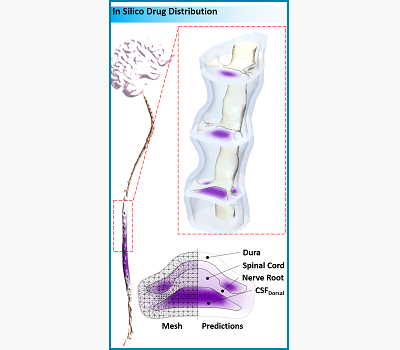
Design of Intrathecal Drug Delivery Systems
Kevin Tangen and Andreas Linninger
Intrathecal (IT) drug delivery is an effective method for delivering therapeutics which cannot cross the blood brain barrier to the central nervous system (CNS) in high doses without causing systemic side effects. Bench-top and animal experiments are generating data to quantify the key factors for the spread and spatial extent of drug distribution. These factors include (i) injection volume and rate, (ii) CSF pulsatility and stroke volume, and (iii) drug binding and uptake kinetics. Rigorous CFD simulations based on these experiments enable the prediction of drug dispersion and tissue uptake in the CNS. Our model offers an in-silico platform for testing and optimization of IT infusion procedures without the need for trial-and-error animal experimentation.
Pain Management, Gene Vector Delivery, and Tumor Treatment: computational and experimental design Heading link
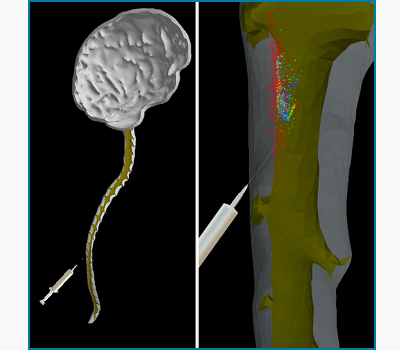
Pain Management, Gene Vector Delivery, and Tumor Treatment: computational and experimental design
Kevin Tangen and Andreas Linninger
To systematically study the spread of species within the CSF spaces a bench-top surrogate was constructed based on anatomical MRI data of a human subject. A precise 3D-printed replica of the entire spinal CSF-filled spaces was fabricated using high resolution medical images of a healthy human volunteer. Additive manufacturing techniques are used to print prototype segments of the spinal CNS. The model is constructed of transparent flexible polymer for the outer dura, and rigid material for the spinal cord, nerve roots, and brain parenchyma. CSF motion is driven by a pulsatile pump which forces fluid into flexible vessels within the cranial space to accurately capture the realistic CSF motion dynamics. Parameters can be varied to study the relative impact on species spread, such as pulse rate and stroke volume. Intrathecal infusion can be administered at any location along the neuroaxis to test clinically implemented treatments and novel infusion strategies.
Conjugation of Therapeutic Drugs on Superparamagnetic Nanoparticle Platform Heading link
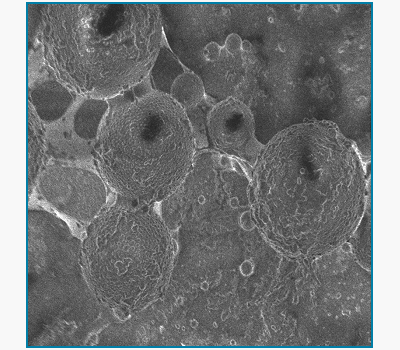
Conjugation of Therapeutic Drugs on Superparamagnetic Nanoparticle Platform
Sebastian Pernal, Indu Venugopal, Wali Badar, Nirav Soni and Andreas Linninger
Magnetic drug targeting is an intrathecal drug delivery technique that uses strong external magnetic fields to localize superparamagnetic nanoparticles at desired locations in the spine or brain. Chemotherapeutic agents are conjugated to the nanoparticle platform so that the nanoparticle-drug complex can be steered to desired target sites by suitably placed magnets. Magnetic targeting maximizes cytotoxicity close to tumor site without systemic drug spread to minimize side effects. Current applications include successful conjugation of the chemotherapeutic agent doxorubicin as well as the anti-inflammatory steroid, dexamethasone. We have also incorporated quantum dots in the nanoparticle platform for in vivo visualization of the drug vehicle conjugates.
Backflow-free catheters for efficient and safe convection-enhanced delivery of therapeutics Heading link
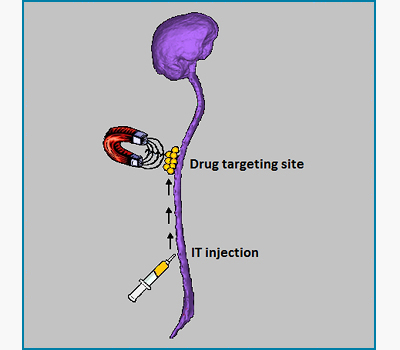
Backflow-free catheters for efficient and safe convection-enhanced delivery of therapeutics
Eric Lueshen, Kevin Tangen, Ankit I. Mehta and Andreas Linninger
Convection-enhanced delivery (CED) is an invasive technique for administering therapeutic drugs directly into the brain tissue to bypass the blood-brain barrier for the treatment of neurodegenerative disorders and brain cancers. Characterization of brain tissue anisotropy with diffusion tensor imaging improves prediction of drug spread in vivo. Optical methods were developed to investigate fluid flow phenomena in soft porous tissues. Our lab designs novel protocols and backflow-free catheters based on in vivo experimental data and computer simulations. Using our novel catheter designs, physicians will be able to target specific regions in high dosages without backflow.
Cerebrospinal Fluid Flow in the Central Nervous System Heading link
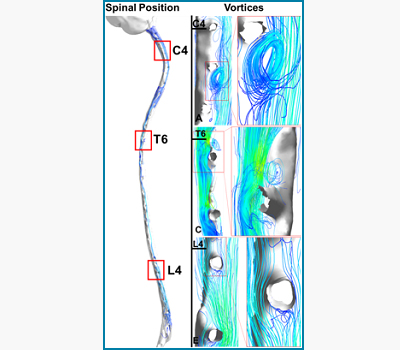
Cerebrospinal Fluid Flow in the Central Nervous System
Kevin Tangen and Andreas Linninger
The pulsatile expansion of the cerebrovascular bed induces cerebrospinal fluid (CSF) flow oscillations in the brain ventricles, as well as the cranial and spinal subarachnoidal spaces. The CSF flow patterns are significantly affected by spinal microanatomical aspects which includes nerve roots and trabeculae. In vivo MR measurements, in combination with detailed computer simulations of CSF flow in the entire central nervous system, are used to elucidate flow profiles in normal subjects and of patients with hydrocephalus. Pulsatile CSF flow is a key factor for drugs dispersion in the CSF.
Intrathecal Magnetic Drug Targeting Heading link
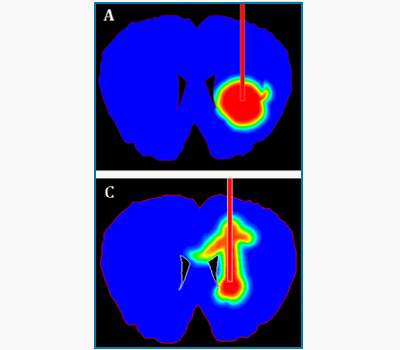
Intrathecal Magnetic Drug Targeting
Indu Venugopal, Sebastian Pernal and Andreas Linninger
IT drug delivery is used to deliver gene therapies, enzymes or therapeutic agents across the blood brain barrier directly to the cerebrospinal fluid. Using external magnetic fields to steer our novel superparamagnetic nanoparticle delivery platform, we have been successful in localizing more than 80% of the entire administered drug to desired target locations in the human spinal canal. Our lab uses in vitro models of the spinal canal and brain as well as small animal models to experimentally demonstrate the efficacy of this technique. Magnetic drug targeting is a promising improvement over conventional IT drug delivery.